Alkalinity is the buffering capacity of a solution. It is a valuable water quality parameter used for many applications, including, but not limited to: drinking water treatment, domestic and industrial wastewater treatment, swimming pools, food and beverage, soil, agriculture, and other environmental testing.
The main compounds of alkalinity are: hydroxides (OH -), carbonates (CO3 2-), and bicarbonates (HCO3 -). The buffering capacity of a solution depends on the absorption of positively charged hydrogen ions by negatively charged bicarbonate and carbonate molecules. When bicarbonate and carbonate molecules absorb hydrogen ions, there is a shift in equilibrium without a significant shift in pH. A sample with high buffering capacity will have high bicarbonate and/or carbonate content, and a greater resistance to changes in pH.
MANTECH complies with EPA Method 310.1, ASTM Method D 1067-92, and Standard Method 2320, for determining alkalinity, which calculates the concentrations of OH -, CO3 2-, and HCO3 -. Total alkalinity is calculated as the sum of these three species and is expressed in units of milligrams of calcium carbonate per litre (mg CaCO3/L).
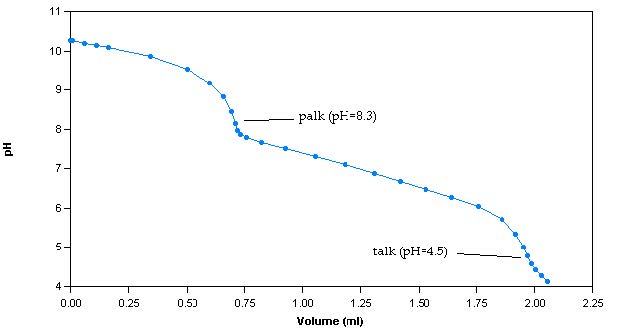
The alkalinity method involves titrating samples with a titrant of known concentration, commonly, 0.02N sulphuric acid (H2SO4), to pH endpoints of 8.3 and 4.5. The alkalinity calculated up to endpoint 8.3 is classified as phenolphthalein alkalinity (palk), whereas the alkalinity calculated up to endpoint 4.5 is classified as total alkalinity (talk). A titration curve is generated by plotting pH versus volume of dispensed titrant (Figure 1).
Figure 1: Sample titration curve for a 100ppm alkalinity sample
The titration curve is used to determine the volume of dispensed titrant at each endpoint. To calculate palk, the following equation is used:
𝑝𝑎𝑙𝑘=𝑥𝑣𝑜𝑙(8.3)× 𝑡𝑐𝑜𝑛×50 000/𝑠𝑣𝑜𝑙
Where:
𝑥𝑣𝑜𝑙(8.3)=𝑣𝑜𝑙𝑢𝑚𝑒 𝑜𝑓 𝑡𝑖𝑡𝑟𝑎𝑛𝑡 𝑢𝑠𝑒𝑑 𝑡𝑜 𝑡𝑖𝑡𝑟𝑎𝑡𝑒 𝑡𝑜 𝑝𝐻 8.3 (𝑚𝐿)
𝑡𝑐𝑜𝑛=𝑛𝑜𝑟𝑚𝑎𝑙𝑖𝑡𝑦 𝑜𝑓 𝑡𝑖𝑡𝑟𝑎𝑛𝑡(𝑁) 50 000= 𝑒𝑞𝑢𝑖𝑣𝑎𝑙𝑒𝑛𝑡 𝑤𝑒𝑖𝑔ℎ𝑡 𝑜𝑓 𝐶𝑎𝐶𝑂3 𝑎𝑠 𝑑𝑒𝑓𝑖𝑛𝑒𝑑 𝑖𝑛 𝑆𝑡𝑎𝑛𝑑𝑎𝑟𝑑 𝑀𝑒𝑡ℎ𝑜𝑑𝑠 (𝑚𝑔/𝑚𝑜𝑙)
𝑠𝑣𝑜𝑙=𝑣𝑜𝑙𝑢𝑚𝑒 𝑜𝑓 𝑠𝑎𝑚𝑝𝑙𝑒 𝑡𝑖𝑡𝑟𝑎𝑡𝑒𝑑 (𝑚𝐿)
talk is calculated using the following equation:
𝑡𝑎𝑙𝑘= 𝑥𝑣𝑜𝑙(4.5)× 𝑡𝑐𝑜𝑛× 50 000/𝑠𝑣𝑜𝑙
Where:
𝑥𝑣𝑜𝑙(4.5)=𝑣𝑜𝑙𝑢𝑚𝑒 𝑜𝑓 𝑡𝑖𝑡𝑟𝑎𝑛𝑡 𝑢𝑠𝑒𝑑 𝑡𝑜 𝑡𝑖𝑡𝑟𝑎𝑡𝑒 𝑡𝑜 𝑝𝐻 4.5 (𝑚𝐿)
𝑡𝑐𝑜𝑛=𝑛𝑜𝑟𝑚𝑎𝑙𝑖𝑡𝑦 𝑜𝑓 𝑡𝑖𝑡𝑟𝑎𝑛𝑡 (𝑁)
50 000= 𝑒𝑞𝑢𝑖𝑣𝑎𝑙𝑒𝑛𝑡 𝑤𝑒𝑖𝑔ℎ𝑡 𝑜𝑓 𝐶𝑎𝐶𝑂3 𝑎𝑠 𝑑𝑒𝑓𝑖𝑛𝑒𝑑 𝑖𝑛 𝑆𝑡𝑎𝑛𝑑𝑎𝑟𝑑 𝑀𝑒𝑡ℎ𝑜𝑑𝑠 (𝑚𝑔/𝑚𝑜𝑙)
𝑠𝑣𝑜𝑙=𝑣𝑜𝑙𝑢𝑚𝑒 𝑜𝑓 𝑠𝑎𝑚𝑝𝑙𝑒 𝑡𝑖𝑡𝑟𝑎𝑡𝑒𝑑 (𝑚𝐿)
For samples with talk less than 20ppm, a low alkalinity titration procedure requires the sample to be titrated to a pH of 4.3, as per Standard Method 2320. To calculate talk of low alkalinity samples, the following equation is used:
𝑡𝑎𝑙𝑘= (2 × 𝑥𝑣𝑜𝑙(4.5)−𝑥𝑣𝑜𝑙(4.2)) × 𝑡𝑐𝑜𝑛× 50 000/𝑠𝑣𝑜𝑙
Where:
𝑥𝑣𝑜𝑙(4.5)=𝑣𝑜𝑙𝑢𝑚𝑒 𝑜𝑓 𝑡𝑖𝑡𝑟𝑎𝑛𝑡 𝑢𝑠𝑒𝑑 𝑡𝑜 𝑡𝑖𝑡𝑟𝑎𝑡𝑒 𝑡𝑜 𝑝𝐻 4.5 (𝑚𝐿)
𝑥𝑣𝑜𝑙(4.2)=𝑣𝑜𝑙𝑢𝑚𝑒 𝑜𝑓 𝑡𝑖𝑡𝑟𝑎𝑛𝑡 𝑢𝑠𝑒𝑑 𝑡𝑜 𝑡𝑖𝑡𝑟𝑎𝑡𝑒 𝑡𝑜 𝑝𝐻 4.2 (𝑚𝐿)
𝑡𝑐𝑜𝑛=𝑛𝑜𝑟𝑚𝑎𝑙𝑖𝑡𝑦 𝑜𝑓 𝑡𝑖𝑡𝑟𝑎𝑛𝑡 (𝑁)
50 000=𝑒𝑞𝑢𝑖𝑣𝑎𝑙𝑒𝑛𝑡 𝑤𝑒𝑖𝑔ℎ𝑡 𝑜𝑓 𝐶𝑎𝐶𝑂3 𝑎𝑠 𝑑𝑒𝑓𝑖𝑛𝑒𝑑 𝑖𝑛 𝑆𝑡𝑎𝑛𝑑𝑎𝑟𝑑 𝑀𝑒𝑡ℎ𝑜𝑑𝑠 (𝑚𝑔/𝑚𝑜𝑙)
𝑠𝑣𝑜𝑙=𝑣𝑜𝑙𝑢𝑚𝑒 𝑜𝑓 𝑠𝑎𝑚𝑝𝑙𝑒 𝑡𝑖𝑡𝑟𝑎𝑡𝑒𝑑 (𝑚𝐿)
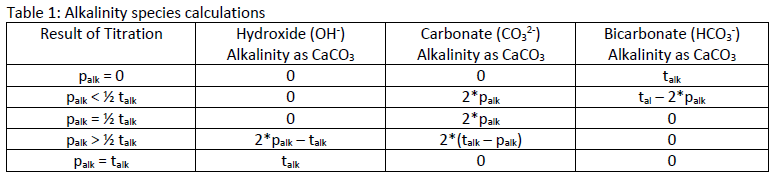
The values for talk and palk can be used to determine the concentrations of OH-, CO32, and HCO3-, and indicate the following conditions:
1. OH – alkalinity is present if palk is greater than half of the talk
2. CO3 2- alkalinity is present when palk is not zero, but is less than talk
3. HCO3 – ions are present if palk is less then half the talk
Table 1 shows the calculations for determining the concentration of each species of alkalinity based on the results for palk and talk.
Table 1: Alkalinity species calculations
MANTECH’s MT Series automates alkalinity and low alkalinity measurements, following EPA, ASTM, ISO and Standard Methods. For more information on how MANTECH can simplify your alkalinity analysis, refer to the MT Series product info page or contact your local MANTECH distributor.
For more information on MANTECH’s method for automated alkalinity measurement and how the species of alkalinity are calculated, download the pdf.