In July 2020, Health Canada released the Guidance on Natural Organic Matter in Drinking Water and most significantly, chemical oxygen demand (COD) was included as a suggested parameters to monitor. With a suggested treated water quality target of <5 mg/L O2, the chemical oxygen demand test method (using potassium dichromate) cannot detect levels lower than 20 mg/L O2. As a result, the guidance references the photoelectrochemical oxygen demand (PeCOD) method with a detection limit of 0.7 mg/L O2 to monitor COD in drinking water.
What is a Guidance?
The intent of a guidance, specifically the Guidance on Natural Organic Matter in Drinking Water, is to provide governments and other stakeholders (such as water system owners, consultants, equipment suppliers and laboratories) with guidance on the impacts of natural organic matter (NOM) on the overall quality of drinking water, including its potential effects on drinking water treatment processes and consequently on the safety of drinking water. Also, this document provides specific guidance on treatment, monitoring, and water quality goals.
What is Natural Organic Matter?
The guidance defines natural organic matter (NOM) as an extremely complex mixture of organic compounds and is found in all groundwater and surface waters. NOM occurs naturally in the environment (both autochthonously and allochthonous) and may also be the result of anthropogenic (human) activities. Anthropogenic sources of NOM include septic systems, wastewater treatment and stormwater discharges, agricultural runoff and industrial discharges. NOM is a critical target for drinking water treatment that is used to protect drinking water quality and public health.
Impact of Natural Organic Matter
Although NOM does not have direct health effects, it critically affects drinking water treatment and can contribute to indirect health impacts, as well as operational and aesthetic issues.
Formation of Disinfection By-Products (DBPs)
Chlorine, one of the most commonly used chemicals to disinfect drinking water, kills potentially fatal bacteria and viruses but comes with unintended consequences. When chlorine is combined with some common NOM compounds, such as trihalomethanes (THMs) and haloacetic acids (HAAs), potentially carcinogenic and/or genotoxic disinfection by-products (DBPs) form. Although other factors can affect DBP formation, in order to mitigate the formation of potentially harmful DBPs, it is important that water utilities understand the source-specific reactivity of NOM when selecting a disinfectant. The removal of NOM is a recommended best practice from Health Canada to minimize the formation of both regulated and non-regulated DBPs. This may require specific monitoring to ensure adequate precursor removal.
Biological Stability
The biological stability of drinking water refers to the concept of maintaining microbiological water quality from the point of production to the point of consumption. NOM encourages bacterial growth and biofilm development in the distribution system and premise plumbing, which can lead to issues that have public health significance. While biofilm microorganisms utilize the constituents with the shortest biodegradation half-lives first, they are adept at consuming all types of available NOM constituents leaving a treatment facility to support their growth in the distribution system. Optimization of NOM removal has been identified as one of several strategies to improve biostability and minimize biofilm development in the distribution system and premise plumbing.
Corrosion Impacts
Although there are a wide variety of factors that can cause corrosion in drinking water distribution systems, NOM has been shown to affect lead and copper corrosion. The effects of NOM on metal surfaces can be varied. NOM can either: 1) complex with calcium ion and prevent protective scale formation; or 2) act as a food source for microorganisms, which could in turn attack the pipe surface and increase corrosion. In practice, Arnold et al. (2012) demonstrated that removing NOM was an effective method to decrease blue-water issues in a school with new copper plumbing. Researchers currently recommend that NOM be removed to minimize lead and copper concentrations.
Effectiveness of the Coagulation Process
Part of the coagulation process involves removing NOM by a phase change that converts dissolved organic matter into particles: either directly by precipitation or by adsorption onto particles created by the coagulant. When metal coagulants are added to the water, chemical reactions occur with both particles and NOM. Therefore, when a coagulant is added, the NOM acts as a ligand that complexes the positively charged metal ions, exerting a coagulant demand that must be overcome before flocculation can occur. As NOM concentrations can rapidly increase four- to five-fold during storm events, it is important that water utilities have a good understanding of NOM’s impact on coagulant dosing. Failure to adjust the coagulant dose in accordance with a change in NOM may contribute to suboptimal coagulation conditions and a decrease in pathogen log removal capability
Effectiveness of Membrane Treatment
NOM has been identified in numerous studies as being responsible for membrane fouling, which can significantly impair water treatment operations. It is generally accepted that the hydrophilic neutral fraction of NOM, comprising polysaccharides and proteins in macromolecular and/or colloidal form (i.e., biopolymers), is responsible for membrane fouling. It is hypothesized that once fouling is initiated by biopolymers, a decrease in electrostatic forces allows hydrophobic NOM to adsorb to the membranes, resulting in further fouling. Although other factors can influence membrane fouling, water utilities should have a good understanding of how the NOM in their source water will interact with membranes to avoid configurations that incur significant fouling.
Aesthetic Concerns
It is well established that NOM is responsible for such aesthetic concerns as colour, taste and odour. Chlorine reactions with NOM may also contribute to tastes and odour. Odour-causing compounds (e.g., terpenoids) and precursors (e.g., amino acids, proteins) are not effectively removed by conventional treatment. Thus, other processes may be necessary to minimize taste and odour. Once odorous compounds are formed, they can persist in the distribution system for up to 500 hours (≈21 days) at 15°C. Concentrations can also increase in the distribution system due to the release of amino acids or peptides from the biofilm.
How to Monitor and Quantify NOM
Throughout this guidance, it is evident of the importance of routinely monitoring the concentration and character of NOM and to evaluate its impact on treatment, water quality and distribution system conditions. The goal of the NOM control strategy should be to always ensure protection from microbial risks, while minimizing DBP, lead and copper concentrations and controlling biofilm formation in the distribution system. The report states that water utilities should have a good understanding of:
- their water source and the nature and generation of NOM;
- whether NOM changes seasonally or with precipitation/snowmelt events; and
- how NOM interacts with treatment processes.
The numerous organic compounds that contribute to NOM cannot be measured directly however, there are a number of surrogates that can be used to provide an indication of the NOM concentration. The most commonly used surrogates include total organic carbon (TOC), dissolved oxygen carbon (DOC) and UV absorbance. Most significantly, Health Canada has added chemical oxygen demand (COD) as a suggested parameters to monitor with a suggested treated water quality target of <5 mg/L O2.
Table 1: Suggested Parameters to Monitor
| Parameter | Location | Frequency: Variable Source | Frequency: Stable Source | Frequency: Ideal |
|---|---|---|---|---|
| Organic colour (true colour) | Raw and treated | Daily | Weekly | Online |
| UV absorbance (at 254 nm) or UV transmittance | Raw and filtered | Daily | Weekly | Online |
| Chemical oxygen demand (COD) | Raw, treatment processes and treated | Daily | Weekly | Online |
| Dissolved or total organic carbon (DOC or TOC) | Raw and treated | Daily | Weekly | Online |
| Specific UV absorbance (SUVA)— calculate from UV254 and DOC | Raw and treated | Daily | Weekly | Online |
|
Inorganic compounds that can enhance the reactivity of NOM to form DBPs:
|
Raw and treated | Weekly | Monthly | Daily |
| Coagulant demand | Coagulation process | Daily | Daily | Online |
| Zeta potential or streaming current— when NOM controls or influences coagulant dose | Coagulation process | Quarterly | Quarterly | Quarterly |
| Disinfection by-products (DBPs) | Distribution system | |||
|
Biological stability:
|
Distribution system | Weekly
Every two weeks
Monthly |
Weekly
Monthly
Monthly |
Online |
Influence of NOM on corrosion:
|
In accordance with corrosion control program | – | – | – |
Table 2: Suggested Treated Water Quality Targets
| Parameter | Units | Source with high specific DBP yield or extensive distribution system | Source with low specific DBP yield |
|---|---|---|---|
| Organic colour | TCU | 5 – 10 | <15 |
| UV absorbance (at 254 nm) or UV transmittance | cm-1 | 0.02 – 0.04 | 0.02 – 0.07 |
| UV transmitter | Percent | 90 – 95 | 85 – 95 |
| COD | mg/L O2 | <5 | <5 |
| DOC – for DBP control | mg/L C | <4 | <4 |
| DOC – for biological stability | mg/L C | <1.8 | <1.8 |
“TOC on its own sheds no light on the oxidizability of the measured carbon or the amount of oxygen needed for its biodegradation” (TOC Manufacturer Brochure).
“Historically, the chemical oxygen demand test method (using potassium dichromate) was not sensitive enough for drinking water (Rittman and Huck, 1989). More sensitive methods have since been developed. One involves using potassium permanganate as the oxidant [ISO 8467]; the other is a photoelectrochemical oxygen demand (PeCOD) method using UV activated titanium dioxide as the oxidant (Zhao et al., 2004; ASTM D8084, 2017)” (Guidance of Natural Organic Matter in Drinking Water, Health Canada, 2020).
“PeCOD® measures the chemical reactivity and associated oxidative changes in Natural Organic Matter (NOM). As a result, it is more sensitive than TOC and UV254 to changing NOM concentrations in source and treated drinking waters” (Centre for Water Resource Studies, Dalhousie University, WQTC 2019).
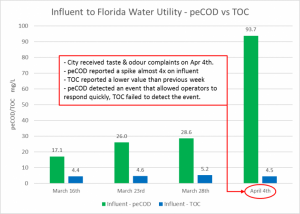
MANTECH’s proprietary PeCOD® analyzer uses UV-activated nanoparticle TiO2 (titanium dioxide) photocatalyst technology coupled with an external circuit to measure the chemical reactivity and associated oxidative changes in natural organic matter (NOM). When a water utility in Cocoa, FL experienced an event triggering taste and odour complaints from residents, PeCOD reported a spike of almost 4x than that of the previous week while TOC reported a lower value. When PeCOD recognized the spike, plant operators were able to act quickly to adjust treatment parameters, mitigate the increase in source water reactivity, rectify the taste and odour event, and control THMs within regulatory limits. For this reason, the PeCOD® is an “optimized” TOC analyzer, providing a critical indication of the oxidizability of the organic carbon in a water sample. The PeCOD is available as benchtop and online analyzer models, plus additional parameters such as alkalinity, pH, conductivity can be added.

Optimize Your TOC & Measure NOM
Read Full Guidance on Natural Organic Matter in Drinking Water