Alkalinity by Titration
Method Abstract #72
Scope and Application
This alkalinity method conforms to Standard Methods 2320 B, ASTM D 1067, EPA 310.1 and both ISO 9963-1 and 9963-2. It determines the total and phenolphthalein alkalinity of aqueous samples, along with measuring the concentrations of carbonate, bicarbonate, and hydroxide. Gran alkalinity can also be determined simultaneously.
Method Summary
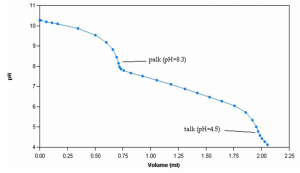
Alkalinity analysis involves the titration of samples with standard 0.02N sulphuric acid (H2SO4) titrant to endpoints of pH 8.3 and 4.5. For alkalinities less than 20 mg CaCO3/L, an additional endpoint at pH 4.2 is recorded. 0.02N hydrochloric acid (HCl) titrant may also be used.
Sample Titration Curve
Method Performance
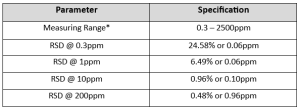
Speciated Alkalinity and Low-Level (<20ppm) Alkalinity according to Standard Methods is automatically calculated and determined with every Alkalinity Titration.
Example Speciation of Alkalinity
*Data for this measuring range was obtained using laboratory prepared standards formulated from sodium carbonate. The measuring range may be increased by using larger capacity analysis vessels and/or auto-dilution.