Biogas Production
As the price of crude oil fluctuates, humans are forced to consider the fact that there is a finite amount of oil on the planet available for our use. Biogas is a secondary energy source derived from renewable resources, which is converted into “end-point energy.” Biogas is a result of anaerobic bacterial degradation of organic matter. Not only does biogas reduce CO2 emissions, but the digestate produced is an arguably better fertilizer compared to the mineral fertilizers currently used. In addition to these benefits, biogas production assists in the treatment and disposal of the ever-growing animal manure produced. The FOS/TAC ratio is one of the most commonly used parameters for monitoring biogas production.
There are 4 main processes in anaerobic digestion. The first step, hydrolysis, transforms organic polymers into smaller chains of sugars, amino acids, and fatty acids. This process can take hours to days. The second step, acidogenesis, produces organic acid intermediates by acidogenic bacteria. Examples of these intermediates include pyruvic acid, butyric acid, acetic acid, and propionic acid. Volatile fatty acids (VOFs) are also produced in this phase. The third step, acetogenesis, uses the products produced during acidogenesis for other bacteria in an endergonic reaction. During this step, several products may be formed depending on the partial pressure of hydrogen in the reaction. During acetogenesis, VOFs are broken down into acetate and hydrogen gas. The last step, methanation, is dependent on the anaerobic conditions of this process. Carbon molecules in the biomass are converted into carbon dioxide and methane. There are several different types of methanogenic bacteria that will degrade different substrates, including CO2 type, methyl type, and acetate type species.
The fermentation process is highly complex and susceptible to interferences. Small changes to temperature or substrate concentration have the capacity to stop a biogas plant for several weeks as the microorganisms adjust to the environment. All steps of this process must be working in synchrony at a balanced rate to ensure the success of the subsequent step. Continuous regulation and monitoring of the parameters of the digester is paramount to the success of the plant. Automatic measuring sensors allow for instant results, but if they are improperly installed, the digester may be disturbed in the process.
COD Application
Measuring the Chemical Oxygen Demand (COD) of substrates prior to fermentation is an important step in calculating the theoretical yield of biogas. A study performed in 2004 by Güngör-Demirci & Demirer found that manure with an initial COD concentration of 53,500 ppm resulted in a higher biogas yield than manure with a COD concentration of only 12,000 ppm. Furthermore, the economic feasibility of using industrial wastewater as a substrate can be assessed by measuring the initial COD of the waste. COD reduction can be converted into theoretical methane production. Even though this is not a true estimate because not all COD is biodegradable, further calculations can be conducted regarding the fractions of COD that are readily, slowly, or non-biodegradable.
FOS/TAC Ratio
Regular measurements of volatile organic acids (FOS) and total inorganic carbon (TAC) (i.e. carbon buffer capacity) have given laboratories the ability to monitor the stability of the digester. The stability of the digestion process can be analyzed by either TAC or FOS; however, it can be better analyzed by the ratio of the 2 parameters. The purpose of using a ratio method is to avoid biased results that may appear when only using one method. Bias in these calculations occurs due to the inhibiting and promoting factors in the fermentation process that prevent one single parameter from indicating its efficacy. To avoid this issue, the FOS/TAC ratio is a commonly applied measurement for observing stability and indicating if corrective action must be taken. FOS/TAC ratio method is also commonly used due to the time consuming and expensive nature of other measurements of the quality of biogas plants.
To find the FOS/TAC value, a representative sample of the substrate is used. All particulate matter must be removed by filtration or centrifugation, and all sample preparation must be performed in the same manner. Typical sample volume of the substrate is 20 mL but may be diluted with deionized water if there is an insufficient amount. Note that the TAC equation must be altered to account for the dilution.
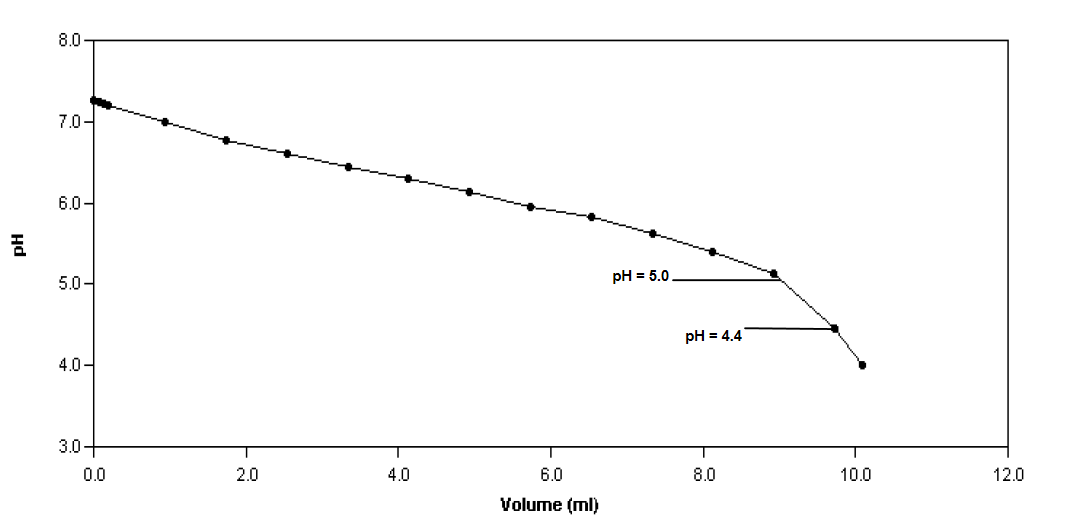
First, TAC is measured by titrating the sample to pH 5.0 with 0.1 N sulphuric acid in mg CaCO3/L and can be calculated by using the following equation:
TAC = (EP1 x Concentration of titrant x 50045) / (Volume of sample)
where EP1 is the volume of the titrant at pH 5.0 in mL. If Ctitrant is 0.1 N and Vsample is 20 mL, the equation can be simplified to:
TAC = EP1 x 250 [mg/L CaCO3]
Following the titration to calculate TAC, the Nordmann method is used to determine the FOS content by titrating a 20 mL sample from pH 5.0 to pH 4.4 using 0.1 N sulphuric acid. Using the following equation where B is the acid consumed in mL (i.e. volume of titrant at pH 5.0 – volume of titrant at pH 4.4).
FOS = [(B x 1.66) – 0.15] x 500 [mg/L HAc]
In general, a FOS/TAC value of 0.3-0.4 is considered optimal; however, every digester has a unique ratio. Above this range, there is excessive biomass input and below this range, there is too little biomass input.
MANTECH Systems
MANTECH manufactures two analyzer tools applicable to biogas applications the PeCOD COD analyzer for COD and BOD tests in a fast (minutes) and safe manner utilizing green method and the MT series Automated Titration Systems for FOS/TAC ratio determination. MANTECH’S PeCOD® COD Analyzer can provide fast and accurate measurements of COD without using harmful chemicals. The PeCOD® community of enduser has surpassed 250 endusers as of March 2019. The PeCOD® COD Analyzer is available in the following configurations:
- Benchtop L50
- Portable L50
- Automated L50 with Multi-Position Autosampler
- Online L50 (Autonomous Real time)
More information about the PeCOD® COD Analyzer can be found at https://mantech-inc.com/analysis-systems/chemical-oxygen-demand/.
MANTECH’s MT-Series Automated Titration Analysis is fully capable of measuring and analyzing the FOS/TAC ratio out of a single sample tube. Laboratory analysts can analyze with standardized, proven software, making the MT-Series an extremely robust machine. There are >2,400 MANTECH analyzers operating in the world measuring >500,000 samples every day. The MT series is available in the following configurations:
- MT-30 Benchtop model, one sample at a time
- MT-30 with Multi-position Autosampler
- Real time analyzer operating autonomously at user set sampling intervals
More information about MANTECH’s MT-Series can be found at https://mantech-inc.com/analysis-systems/automated-titration-analysis/.
The MANTECH PeCOD and MT series may also be combined into a single analyzer for multi-parameter testing capability.
The following shows a sample calculation of the FOS/TAC ratio attributed to the sample analyzed in Figure 1. At pH 5.0, the volume of titrant added is 8.97 mL, and at pH 4.4 it is 9.81. Therefore, the value of B is 0.84.
The FOS/TAC ratio of this substrate is slightly below what is generally considered the optimal level.
FOS/TAC = {[(B x 1.66) – 0.15] x 500} / (EP1 x Concentration of titrant x 50045) / (Volume of sample)
FOS/TAC = {[(0.84 mL x 1.66) – 0.15] x 500} / (8.97 mL x 0.1 N x 50045 / 20 mL)
FOS/TAC = 0.28
References
Dieter, D. & Steinhauser, A. (2010). Biogas from Waste and Renewable Resources: An Introduction, 2nd Ed. Weinheim, Germany: Wiley‐VCH.
Güngör-Demirci, G. & Demirer, G. N. Bioresource Technol. 2004, 93(2), 109-117.
Lethbridge Biogas, 2019. [Online] accessed August 8, 2019. http://www.lethbridgebiogas.ca.
Li li, M., Biro, G., Sulyok, E., Pais, M., Borbely, J., Tamas, J. (2011). Novel approach of the basis of FOS/TAC method. Debrecen, Hungary.
Lossie, U. & Pütz, P. (2009). Targeted control of biogas plants with the help of FOS/TAC: Practice Report. Mechelen, Germany.
Welland, P. Appl. Microbiol. Biotechnol. 2010, 85(4), 849-860.
Author: Laura Martin, Quality Control and Research Chemist