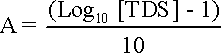Scaling is defined as the deposition of mineral solids on the interior surfaces of water lines and containers. Many factors can affect scaling, but it most often occurs when water containing the carbonates or bicarbonates of calcium and magnesium is heated. Encrustation of tubing, boilers, coils, jets, sprinklers, cooling towers, and heat exchangers arise wherever hard water is used. Scale formation can greatly affect heat transfer performance. One mm thick scale, for example, can add 7.5% to energy costs, while 1.5 mm adds 15% and 7 mm can increase cost by over 70%!
The Langelier Saturation Index (LSI) is one of the most widely used indicators of cooling water scale potential. This index indicates the driving force for scale formation and growth in terms of pH as a master variable. In order to calculate the LSI, it is necessary to know the alkalinity (mg/L as CaCO3 or calcite), the calcium hardness (mg/L Ca2+ as CaCO3), the total dissolved solids (mg/L TDS), the actual pH, and the temperature of the water (oC). The TDS can be determined using a conductivity measurement.
LSI is defined as:
![]()
Where:
pH is the measured water pH
pHs is the pH at saturation in calcite or calcium carbonate and is defined as:
When interpreting results, the LSI indicates three situations:
-
If LSI is negative: No potential to scale, the water will dissolve CaCO3
-
If LSI is positive: Scale can form and CaCO3 precipitation may occur
-
If LSI is close to zero: Borderline scale potential
In summary, scaling is expected to occur when the LSI is greater than zero, and the more positive the LSI is, the more potential for scaling to form.
MANTECH provides the ideal solution for determining the LSI of cooling water and other waters with the MT100 Analyzer. The MT100 combines all required tests (pH, Alkalinity, Calcium Hardness, TDS by Conductivity) in one user-friendly, convenient solution that is able to perform the entire LSI analysis from a single 50mL sample tube. Contact MANTECH for more information and pricing!