Ca and Mg Hardness with TRIS buffer
Method Abstract #126
Scope and Application
This method is useful for monitoring the calcium, magnesium and total hardness in water samples. It is a variation of Standard Method 2340-C and ASTM D 1126.
Method Summary
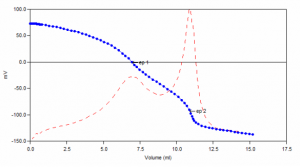
Hardness analysis typically involves the addition of an ammonium hydroxide buffer to a water sample prior to titration. The traditional buffer is caustic and causes a shocking effect on the pH electrode. It also has an undesirable odor. This method utilizes a TRIS-based buffer which eliminates the shocking effect. Like the traditional method, after addition of the buffer samples are titrated with EDTA to two endpoints. From these, the calcium, magnesium, and total hardness concentrations are calculated.
Sample Titration Curve
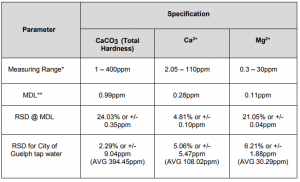
Method Performance
Data for this measuring range was obtained using laboratory prepared standards formulated from calcium carbonate. The measuring range may be increased by using larger capacity analysis vessels and/or auto-dilution.
**The Method Detection Limit (MDL) calculation procedure was obtained from US EPA 40 CFR Appendix B to Part 136 – Definition and Procedure for the Determination of the Method Detection Limit. Results may differ depending on laboratory practices and sample matrixMethod Performance