Acidity by Titration
Method Abstract #88
Scope and Application
This method conforms to Standard Method 2310 B and ASTM D 1067. The acidity of a water sample is its quantitative capacity to react with a strong base to a designated pH. The acidity of a solution is based on the total acidic constituent of a solution.
Method Summary
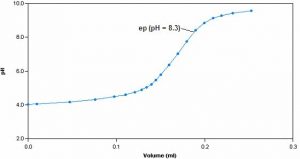
This method involves the titration of a sample with standardized sodium hydroxide to endpoints of pH 3.7 and 8.3, signifying the “methyl orange acidity” and the “phenolphthalein” or total acidity respectively. Since acidity represents a solution’s specific properties rather than a specific chemical’s concentration, it is measured as an equivalent amount of calcium carbonate.
Sample Titration Curve
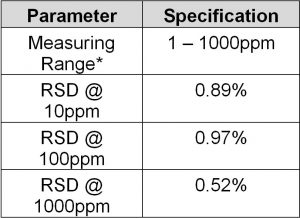
Method Performance
*Data for this measuring range was obtained using laboratory prepared standards formulated from potassium hydrogen phthalate. The measuring range may be increased by using larger capacity analysis vessels and/or auto-dilution.
**The Method Detection Limit (MDL) was determined based on data obtaining a coefficient of variance better than 30%. Results may differ depending on laboratory practices and sample matrix.
RSD values are better than those specified in Standard Methods