Alkalinity and Hardness by Potentiometric Titration Using TRIS Buffer
Method Abstract #97
Scope and Application
This method determines the alkalinity and total hardness of a watersample taken from a single aliquot. It conforms to EPA Method 310.1, Standard Method 2320 and ASTM Method D 1067 for alkalinity analysis, and is a variation of Standard Method 2340, EPA 130.2 and ASTM D 1126 for the hardness measurement.
Method Summary
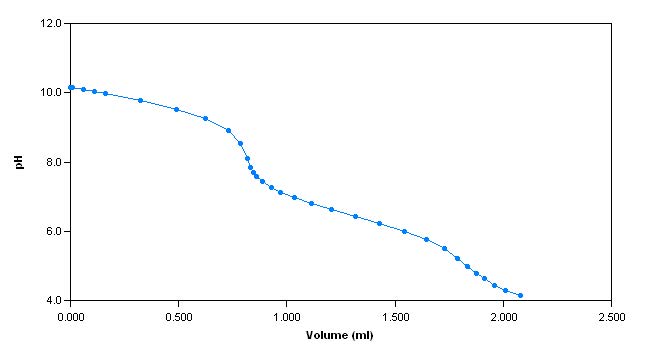
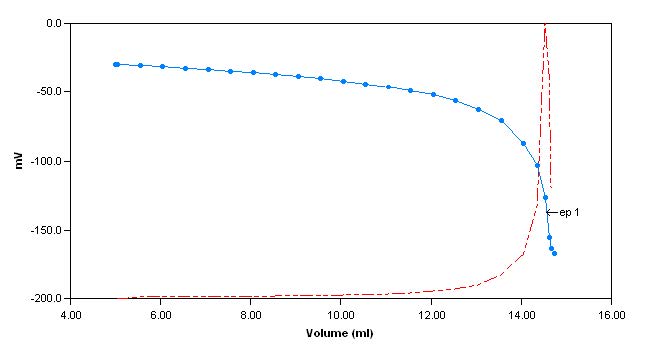
The sample is first titrated with s ulphuric acid to a pH of 4.2. The total alkalinity of the sample is then calculated and reported. Next, pH 10
hardness buffer is added to the sample until the pH of the sample reaches 10. The sample is then titrated with EDTA to an endpoint (measured with a calcium electrode) and the total hardness is calculatedand reported.
Sample Titration Curves
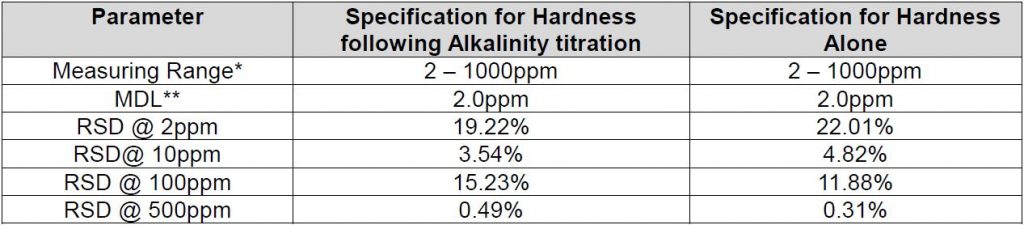
Method Performance
Example Speciation of Alkalinity
*This measuring range was determined by analyzing laboratory-prepared standards formulated from calcium carbonate.
**The Method Detection Limit (MDL) was determined based on data obtaining a coefficient of variance better than 30%. Results may differ depending on laboratory practices and sample matrix