Chlorine by Sodium Thiosulfate Titration
Method Abstract #69
Scope and Application
This method conforms to Standard Method 4500-Cl B, EPA 330.3 and is a variation of ASTM D 1253. It measures the concentration of chlorine in a water sample.
Method Summary
Chlorine concentration is determined by titration with sodium thiosulfate using a redox electrode. Acetate buffer and potassium iodide are added to the sample, leading to the formation of iodine upon reaction with chlorine. The iodine in the sample is then titrated directly with sodium thiosulfate, and is proportional to the concentration of chlorine in the original sample.
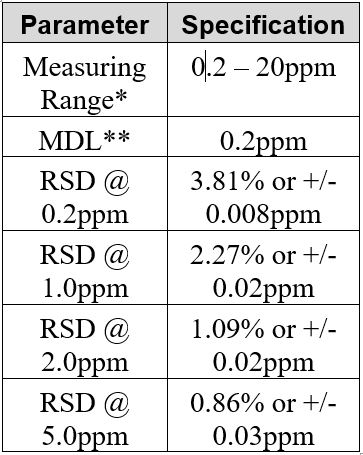
Sample Titration Curve
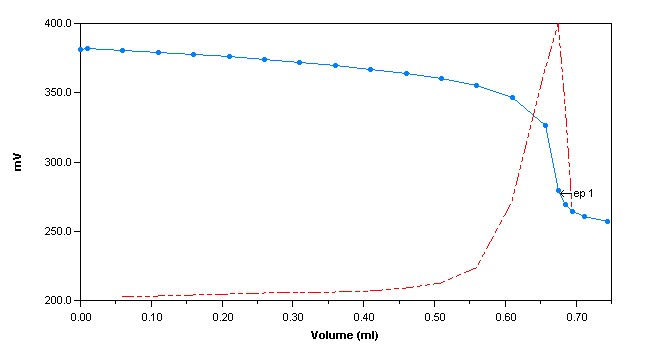
Method Performance
*Data for this measuring range was obtained using laboratory prepared standards formulated from potassium chloride. The measuring range may be increased by using larger capacity analysis vessels and/or auto-dilution.
**The Method Detection Limit (MDL) was determined based on data obtaining a coefficient of variance better than 30%. Results may differ depending on laboratory practices and sample matrix.