Determination of Chloride by Titration
Method Abstract #71
Scope and Application
This method conforms to Standard Method 4500-Cl– D, and is a variation of ASTM D 512 (B) and ISO 9297. This method determines the chloride concentration of water samples.
Method Summary
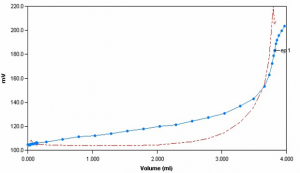
Chloride concentration is determined potentiometrically by titration with silver nitrate using a silver/sulfide ion selective electrode. The reaction involves the precipitation of silver chloride when chloride anions are combined with silver cations. The titration is complete when all the ions are consumed, signified by a large inflection.
Sample Titration Curve
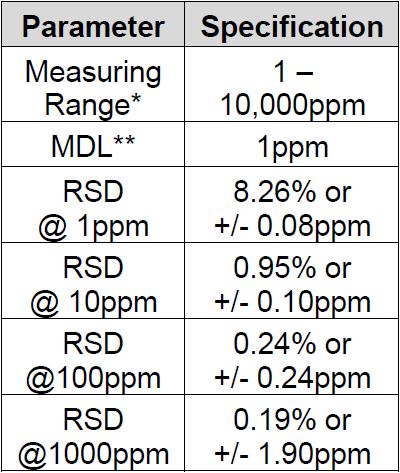
Method Performance
*Data for this measuring range was obtained using laboratory prepared standards formulated from potassium chloride. The measuring range may be increased by using larger capacity analysis vessels and/or auto-dilution.
**The Method Detection Limit (MDL) was determined based on data obtaining a coefficient of variance better than 30%. Results may differ depending on laboratory practices and sample matrix.
RSD values are better than those specified in Standard Methods.