Gran Alkalinity
Method Abstract #128
Scope and Application
Gran alkalinity method is a more accurate technique used explicitly for low level alkalinity samples. It is performed on samples with a pH less than 7.0.
Method Summary
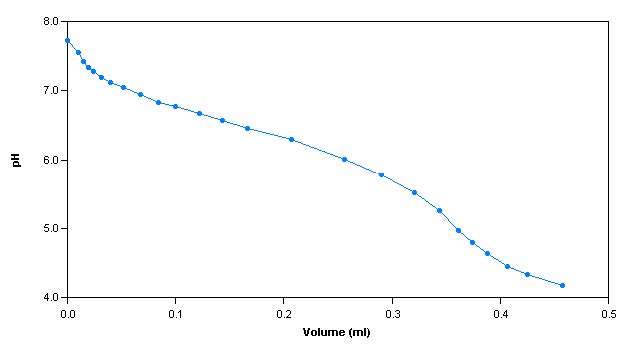
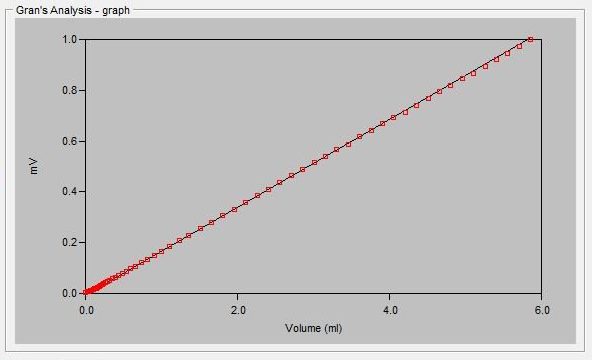
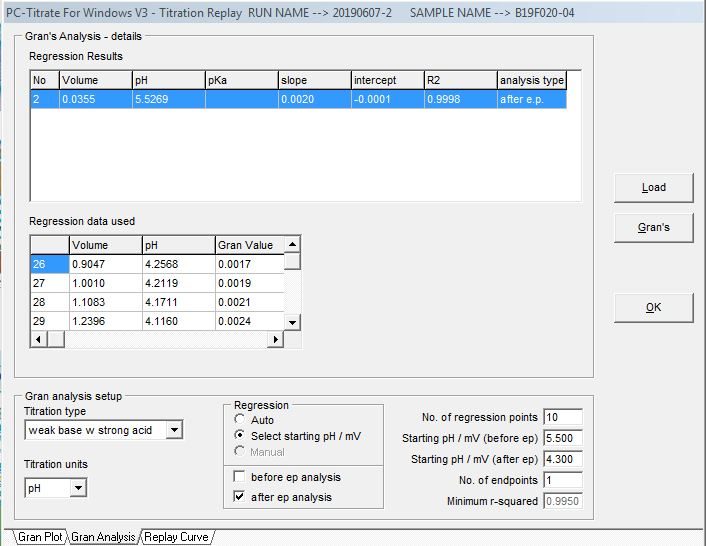
The alkalinity titration is carried well past the total inflection point to about pH 3.0. A linear mathematical extrapolation of the titration curve is performed and is used to calculate the equivalence point.
Sample Titration Curve and Gran Plot
Gran Correlation
Related Posts